“Glass, Science, Definition: The Never-Ending Struggle” Stained Glass Quarterly Vol. 111, No. 4 Winter 2016. 48-53.
Authors: William LaCourse, Ph.D. & Megan McElfresh
Glass is one of nature’s oldest inventions, yet humans are only beginning to understand and exploit its full potential. It may be visually transparent, colored or clear, but scientifically opaque with a host of internal secrets and anomalies. Thus, while artists can capture and tame its beauty, scientists still struggle to fully understand the nature of the glassy state. Physicists and glass scientists still ask the question, “What is glass?”, and have lively discussions with references to an esoteric Biblical verse Judges 5:5 where Deborah stated that “The mountains flowed down at the presence of the LORD, even yon Sinai at the presence of the LORD, the God of Israel.”
In medieval alchemy, the symbol for glass was an infinity above a cross: never ending struggle. Some could argu

e that even in this day and age, not much has changed. Glass is a mysterious, romantic material. The history of glass is full of myths and legends, secrets and hearsay handed down through the ages. Scientifically, molecularly, what is this incredible material that has entranced so many?
As professionals, how many of us can actually define what glass is scientifically? In our organization’s own publication “The Story of Stained Glass” (I have the seventh edition, published 1984) on the second page we address the Substance and Nature of glass in the following way:
“Webster solemnly informs us that glass is an amorphous substance consisting ordinarily of a mixture of silicates…
Webster gives us a key to the wonderful possibilities of glass in his use of the term amorphous. In the hands of master craftsmen this apparently hard and brittle material is capable of taking on elusive qualities of brilliance and movement. Stained glass comes alive…
The very nature of the materials and processes of glass-making are conducive of mystery. Opaque substances are transformed into a completely different material; one isn’t quite sure how. The dull sand, lime & soda that went into the furnace comes out glowing and vibrant, to be controlled only by the greatest skill. It is still sparkling and elusive when it has been cajoled into its final shape.
In a modern world of plastics, it is discovered that glass is the ancient and original plastic. It is not a crystal, but in its formation, is as near like water as anything else…”
Even at the Corning Museum of Glass, I cannot find a definition any better. And in that history of a void where there is no simple definition to fill in that blank, there has been some wiggle room for theories such as “glass is a super-cooled liquid” to take hold.
I suspect that at some point in your own history with glass, very early on, in fact, you were quite simply seduced by the material. At some point when you started working with the material, did your teacher or mentor attempt to explain what glass actually was? What was its chemical makeup? How did it work? Somewhere along the way, somebody out there might have told you some variation of the story: “The reason some pieces of cut glass in the windows we’re restoring is thicker at the bottom than at the top is because at the molecular level glass is still liquid and its being pulled down by gravity.”
– Insert incredulous silence here –

I’m reminded of a line given to Galadriel in the prologue of Lord of the Rings: Fellowship of the Ring, “…And some things that should not have been forgotten were lost. History became legend. Legend became myth…” Even today, in the world of Google and YouTube, I still hear this theory sneak into conversations which are perfectly professional in every other way. Can you imagine the difficulties we would have restoring the world’s stained glass windows, let alone creating new work for architects today if this was true? And yet this sticky theory continues to follow some of us around…
So how instead is glass defined? What – molecularly, definitively – is this amazing material that we use every day, forging light itself into the most brilliant works of art?
As artists and professional glass users, we can serve our industry best if we precisely understand our material! I turned to Dr. William C. LaCourse, Professor of Glass Science at Alfred University’s Inamori School of Engineering, to help me find the answer. His short answer? “Beer is a Super Cooled Liquid… Glass?… not so much.”
What about the definition we get from the dictionary? Unfortunately, Dr. LaCourse points out that just as our Webster definition at the beginning of our article, today’s on-line Dictionary.com definition just leaves too much wiggle room to be a scientifically accurate definition: “a hard, brittle, noncrystalline, more or less transparent substance produced by fusion, usually consisting of mutually dissolved silica and silicates that also contain soda and lime, as in the ordinary variety used for windows and bottles.” Dictionary.com describes rather than defines what glass can sometimes be and how it is sometimes made. It somewhat sneakily does not specify whether glass is a liquid or a solid – it’s a ‘substance.’ Further it implies that the glass is only made by ‘fusion’ – this is too limiting since glass can be made in a number of ways in additional to fusion. And in more and more cases, the glasses DO NOT contain silica or silicates; some commercial glasses today may have no silica or other silicates.
While most people could care less, it is important for a scientist to have a precise but inclusive definition that a material must fulfill to be a glass. (And to do this, we might need that super cooled liquid mentioned above, perhaps conveniently in a chilled glass?) While a definition may still be slightly out of reach, glass can be described in known terms to help us better understand its mystery. Terms such as ‘supercooled liquid,’ ‘glass transition,’ ‘relaxation time,’ ‘fictive temperature,’ and ‘non-crystalline solid’ are parts to the whole of the glassy state. These terms are why conversations about annealing, coefficients of expansion and viscosity are so important, even in the world of glass + art.
Because of the increasing popularity of glass kilnforming as a technique in many of our studios, you probably know more about the science of glass than you think you do. And it probably doesn’t take much for you to take a jump with us into why the definitions above just don’t work.
For a scientist, it is better to define a material in terms of structure and properties without specifying composition similar to the way we describe “matter” as a SOLID, LIQUID, or GAS without specifying composition. Some glass scientists would consider glass as a 4th state of matter. (or, if you are a physicist a 6th state of matter as they consider “plasmas” and the ever popular “Bose-Einstein condensates (BEC) as the 4th and 5th states. Google that to find some delightfully nerdy talking points for future conversations with your super-cooled beer…) Glass is a rigid material formed by heating a mixture of dry materials (batch) to a viscous state, then cooling the ingredients fast enough to prevent formation of the molecules into a regular crystalline structure, but slow enough to prevent shock from internal stress. As the glass cools at a specific rate, the atoms become locked in a disordered state like a liquid before they can form into the perfect crystal arrangement which usually defines a solid. Glass shares the qualities of both states of matter.
Here is another way to think about it: the glass that we use only exists, as a material, under specific conditions, within specific temperature ranges. And it doesn’t behave like any other material while it’s here with us.
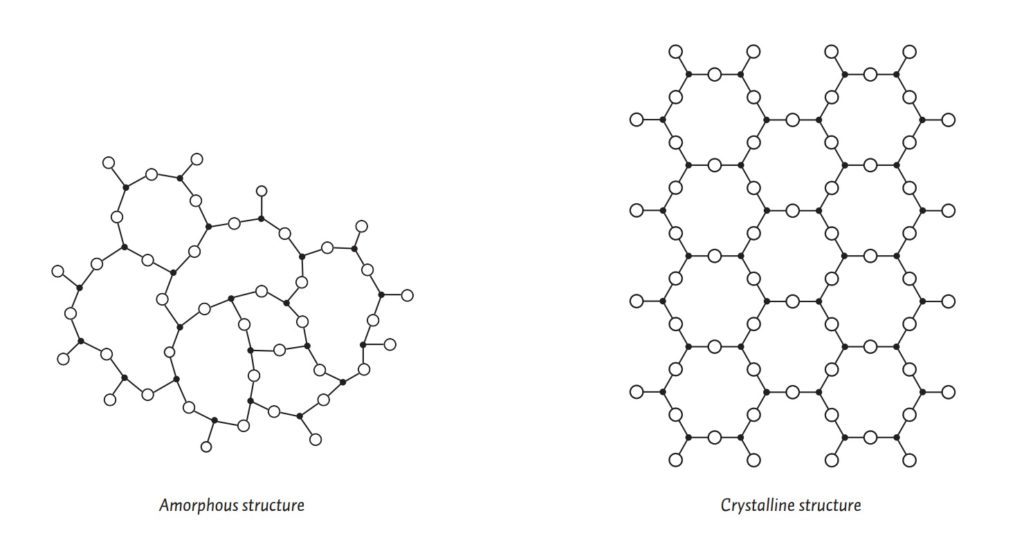
Glass can be “crystal clear” but it can never exist with a “crystalline structure.” Glass is a material that only exists with an amorphous structure. Its molecules are arranged at random — yes, like a liquid — versus a regular, repetitive, specific pattern.
What would a glass look like with a crystalline structure? Well, perhaps once upon a time something overheated in your kiln and your piece devitrified? This is how Corning’s line of Pyrex glass-ceramics came to exist: The story goes that in the early 50’s while developing glass cookware, S. Donald Stookey of the Corning Research and Development Division overheated a sample in the furnace, which turned the sample a milky white color. As he attempted to move the glass from the furnace into the garbage, the sample slipped and hit the floor, but did not shatter. Hence Pyroceram was born, but due to the heat, it was no longer a glass because its molecular structure was no longer amorphous.
You can imagine how confusing it would have been to the first viewers to discover that this hard, brittle material which shattered on impact had a molecular structure that resembled frozen water. It is this molecular structure which earned glass the descriptive term of ‘supercooled liquid.’ It is perhaps less confusing to call glass a ‘non-crystalline solid.’
Now let us examine that whole idea of ‘liquid’ a little closer, because even when its being manufactured, we can all agree that glass never really sloshes around like a glass of water. At its hottest, glass moves like a honey at best. Because it has an amorphous molecular pattern, glass reacts to heat differently than other materials. It does not suddenly go from hard and brittle to soft and honey-esque, it’s a slow change which occurs between specific temperature ranges and it’s the reason why annealing is such a crucial part of both glass manufacture ring and later glass artwork.
Materials like metal, wax or ice change from a solid to a liquid at a very specific temperature (a melting point), whereas materials like glass go through a very gradual transformation—slowly changing from a material that behaves like a solid to a material that behaves like a liquid. Notice the word behaves: this behavior is unique to amorphous materials and is called glass transition from a hard and relatively brittle “glassy” state into a viscous or rubbery state as the temperature is increased.
It is this slow transformation, this behavior of glass transition, which allows glass to be worked cold (cut, ground, sandblasted), warm (kilnformed or fused), or hot (blown, cast, torchworked).
What is happening during this slow transition of heating and cooling is that the chemical bonds within the glass holding the atoms together are breaking apart. The chemical bonds in a regular crystalline structure are identical and so all of the bonds holding the atoms together break at exactly the same temperature. Below this temperature, the material is solid, above this temperature, the material is a liquid, hence, melting point. An amorphous molecular pattern, such as the one found in glass in its rigid state, has chemical bonds across a range of strengths because the molecules are held apart at a range of distances therefore it takes a range of temperatures to break the bonds apart.
Likewise, when cooling glass, it needs a range of time, carefully calculated based on the complexity of the piece, for these molecules to cooperate without undue stress. The process of slowly cooling glass sheets or a completed glass object prevents strain in these varied chemical bonds.
To properly anneal glass, the temperature of the piece is raised just so that the molecular bonds can be relaxed, but not broken. Or the temperature is lowered such that bonds can slowly form, but are not yet stiff. The temperature from the center of the glass mass out to all of its surfaces and extremities needs to be kept as close to the same as possible while it passes through the glass transition state. Ideally, the entire object is at the same uniform temperature. The temperature, which is held high for some time, is then slowly reduced to room temperature.
All these factors come into play when we apply paints and stains to the glass surface and apply heat. It is this amazing material and the slow, ordered process that it demands that allows for silver stain to react so very differently across different glasses of different manufactures. Even the slightest change in the mixtures of silica, metals or oxides can drastically change where these changes begin and the range in which they will occur which is why compatibility is not a simple issue which can be summarized by the “coefficient of expansion.” But that’s a conversation for the next glass of super-cooled liquid.
So, in conclusion: in the day to day work of art glass craftsmen, is glass so solid that it will not flow, even on God’s time scale?
Yes.
Is it a terrible thing that glass remains just outside the borders of clear, concise definition?
Of course not. It is because of the elusive nature of the material itself that we as artists have so much room to play. The future of glass, the full capacity of glass, is yet to be discovered by scientists and artists alike.

Leave a Reply